Everything you wanted to know about Fuel Cells, but were afraid to ask
Feb 12, 2020
Should the discerning Kahn customer be looking at other technologies to power their investment into the future? As a follow up to a feature on electric cars, staff writer Carl Pickles took a look at the other great hope for keeping your car on the road: the hydrogen fuel cell.
For the inquisitive amongst us: a fuel cell is an electrochemical energy conversion device which converts hydrogen and oxygen into water, producing electricity. Putting it simply, it’s like a battery where the chemicals are constantly replenished. The hydrogen and oxygen constantly flow into the cell so it never runs out, providing DC (direct current) that can be used to power motors, lights or anything else electrical.
Petrol engines burn fuels and use the pressure created by expansion of the gases released to carry out mechanical work. Batteries convert chemical energy into electrical energy when needed. Essentially, fuel cells should do both tasks more efficiently.
The different types of fuel cell are classified by their operating temperature and the electrolyte they use. Since they’re the type most likely to be used in cars, we’ll focus on the Polymer Exchange Membrane Fuel Cell (PEMFC).
Get ready for the Science bit:
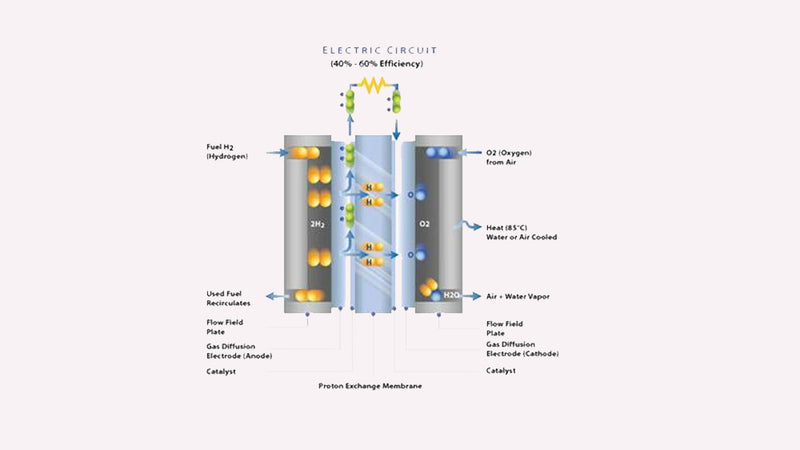
The PEMFC has four basic elements:
The anode (-ve) conducts the electrons that are freed from the hydrogen molecules so that they can be used in an external circuit (such as the one the motor would be part of)The cathode (+ve) conducts the electrons back from the external circuit to the catalyst, where they can recombine with the hydrogen ions and oxygen to form water.The electrolyte is the proton exchange membrane. This specially treated material, which looks something like cling film, only conducts positively charged ions. The membrane blocks electrons. This membrane must be wet to function and remain stable.· The catalyst is a special material that speeds up the reaction of oxygen and hydrogen. It is usually made of platinum nanoparticles coated very thinly onto carbon paper or cloth. The catalyst is rough and porous to maximize the exposed surface area of the platinum. The platinum-coated side of the catalyst faces the PEM.
Petrol and Battery Power Efficiency
If we look at the whole cycle, the efficiency of an electric car is 72% for the car, 40% for the power plant and 90% for charging the car. That gives an overall efficiency of 26%. Not good. But if the electricity is generated by a renewable energy source, then it is basically free, and the efficiency goes back up to 65%.
Scientists are working to boost fuel cell efficiency. One approach is to combine fuel cell and battery-powered vehicles. Ford Motors and Airstream are developing a concept vehicle powered by a hybrid fuel cell drive train named the HySeries Drive. Ford claims the vehicle has a fuel economy comparable to 41 mpg, using a lithium battery to power the car, with the fuel cell recharging it.
Fuel Cell Problems
Cost
They’re expensive. Very expensive. For PEMFC systems, proton exchange membranes, precious metal catalysts (usually platinum), gas diffusion layers, and bipolar plates make up 70% of the cost. To compete with petrol engines, fuel cell systems must cost less than £30 per kilowatt. Currently, the projected high-volume production price is more than £60 per kilowatt. Researchers need to cut the amount of platinum used, or find a new catalyst.
Durability
PEMFC membranes need to be durable and be able to operate at temperatures above 100°C and below 0°C. A 100°C temperature target is needed for a fuel cell to have a higher tolerance to fuel impurities. This is a problem because, as mentioned before, the membranes need to be kept wet. Water freezes at 0°C and starts to boil at above 80°C unless pressurized. Drivers start and stop a car relatively frequently, so the membrane has to be stable under cycling conditions. Currently membranes tend to degrade while fuel cells cycle on and off, particularly as they get hot.
Storage and Other Considerations
A petrol car can usually go something like 300 miles between fill-ups. To create a comparable result with fuel cell vehicles, researchers must overcome hydrogen storage problems. While PEMFC systems have become lighter and smaller as improvements are made, they still are too large and heavy for use in standard vehicles. There are also safety concerns related to fuel cell use. New procedures for fire, police and paramedics to follow when they handle an incident involving a fuel cell vehicle will be needed. Engineers will also have to design safe, reliable hydrogen delivery systems.
Why Use Fuel Cells?
Billions have been spent on research and development. A hydrogen infrastructure will cost considerably more to construct and maintain (some estimates top £375,000 million for the US). So why is this technology worth the investment?
Very simply: Oil. Oil prices are expected to continue to rising over the next few decades as more low-cost sources are used up. Oil companies will have to look in increasingly challenging environments for oil deposits, which will drive oil prices higher.
Fuel cell technologies are an attractive alternative to oil dependency. The only by-product they produce is water. Though engineers are concentrating on producing hydrogen from sources such as natural gas for the short-term, the Hydrogen Initiative has plans to look into renewable, environmentally-friendly ways of producing hydrogen in the future.
Scientists and manufacturers have a lot of work to do before fuel cells become a practical alternative to current energy production methods. Still, with worldwide support and cooperation, the goal to have a viable fuel cell-based energy system may be a reality in a couple of decades.
More Stories >





